is water polar or nonpolar
In typical reactions the alkoxy OR group of an ester is replaced by another group. In summation water is a polar compound formed from two hydrogen atoms and one oxygen atom.
 |
| Why Is Water A Polar Molecule Socratic |
They are composed of atoms that have a negligible difference in electronegativity.
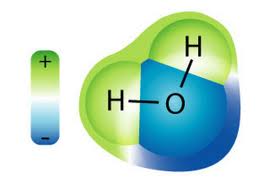
. For example if you want to mix an ionic compound or polar compound in an organic solvent you may be able to dissolve it in ethanol polar but not by a lot. When you look at a diagram of water see Fig. Is water polar ionic or nonpolar. Water is a polar molecule.
Yes water H2O is polar. Water is polar because of its shape. While the H-O-H bond forms a 109 degrees angle the oxygen atom is more electronegative. Each O-H bond in the H 2 O molecule is polar due to a high electronegativity difference between the bonded atoms.
3-2 you can see that the two hydrogen atoms are not evenly distributed around the oxygen atom. Check whether individual bonds are polar or nonpolar The chemical bonds can be either nonpolar polar or ionic depending on the difference of the electronegativity. And how can you say that H2O is a polar molecule. It is composed of two hydrogens H atoms bonded to the central oxygen O atom.
The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two poles a positive. The prime difference between polar. Water H2O is a polar covalent molecule as is hydrogen fluoride HF. The most prominent example is the water molecule.
Difference between polar and non-polar solvents. While the overall charge of the molecule is neutral the orientation of the two positively charged hydrogens 1. Water is a polar molecule meaning that there is an uneven distribution of electron density. In the article that follows youll learn all about the polarity of water.
Nonpolar solvents have nonpolar bonds. Water H2O is a highly polar molecule. This is because of the bent shape of the water molecule due to which there is an unequal charge distribution over the atoms of hydrogen and oxygen involved in the. Examples of polar solvents are.
One such reaction is hydrolysis literally splitting with water The hydrolysis of esters is. Additionally water molecules adopt a. A water diagram see Fig shows that the two hydrogen atoms are not evenly distributed around the oxygen atom. Water is a Polar Covalent Molecule Water H2O like hydrogen fluoride HF is a polar covalent molecule.
Why water makes a good. Water is polar because it has a bent geometry that places the positively-charged hydrogen atoms on one side of the molecule and the negatively-charged oxygen atom on the other side of the molecule. The more electronegative oxygen atom exerts an unequal pull on the molecules constituent. Polar solvents have polar bonds.
Water is one of the most. In contrast water is a polar compound due to its bent structure and dipole moment cannot get zero. Water H 2 O is a polar molecule. H2O Water is a POLAR molecule.
Many bent molecules are in fact polar. Want to know the reason. The polarity reflects the balance between a polar component OH and a non-polar hydrocarbon component existing in the same molecule. Water has a partial negative charge near the oxygen atom due the.
Its helpful to know which compounds are intermediate between polar and nonpolar because you can use them as an intermediate to dissolve a chemical into one it wouldnt mix with otherwise. Is H2O Polar or Nonpolar. If hydrocarbon character increases. The net effect is a partial dipole where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge.
Due to the difference in electronegativity between an O and an H. Lets dive into it. Water is a Polar Covalent Molecule. H2O or Water is a POLAR.
 |
| Polar Covalent Bonds Teaching Chemistry Study Chemistry Chemistry Lessons |
 |
| Water The Polar Molecule Science With The Amoeba Sisters |
 |
| Chemical Polarity Wikipedia |
 |
| Chemical Polarity Wikipedia |
 |
| Solved Draw The Structure Of Water Is Water Considered Polar Or Nonpolar Why |
Posting Komentar untuk "is water polar or nonpolar"